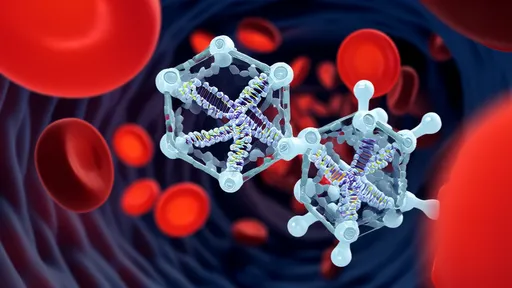
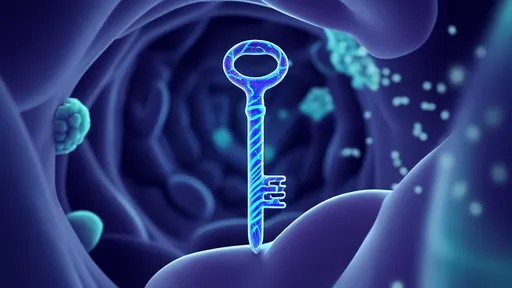
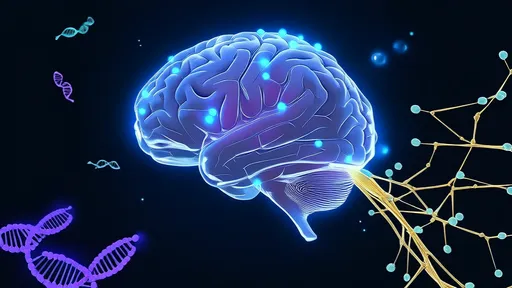
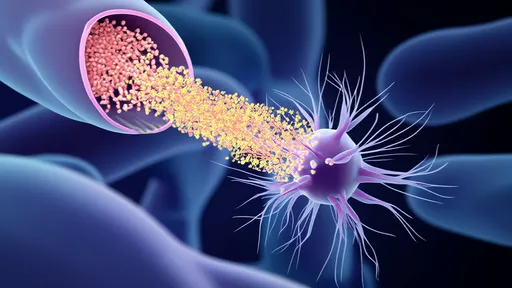
In a groundbreaking advancement that could revolutionize drug delivery to the brain, researchers have harnessed artificial intelligence to design protein-based "smart keys" capable of penetrating the blood-brain barrier (BBB). This biological fortress, which protects the brain from harmful substances, has long been a formidable obstacle in treating neurological disorders. The new approach combines computational biology with deep learning algorithms to create tailored carriers that may finally unlock targeted therapies for Alzheimer's, Parkinson's, and brain cancers.
The blood-brain barrier's selective permeability has frustrated pharmaceutical developers for decades. While essential for keeping toxins and pathogens out of the central nervous system, this network of tightly packed endothelial cells blocks nearly 98% of small-molecule drugs and 100% of large-molecule therapeutics. Traditional solutions like brute-force chemical modification or invasive procedures often compromise efficacy or pose significant risks. The AI-designed protein carriers offer an elegant biological solution inspired by nature's own BBB-crossing mechanisms.
At the core of this innovation lies a machine learning framework trained on thousands of known protein structures and their transport properties. By analyzing how certain viral vectors and naturally occurring peptides navigate the BBB, the system identifies topological and biochemical patterns associated with successful passage. Researchers then use these patterns to generate novel protein sequences optimized for both penetration and payload delivery. Unlike random screening methods, this computational approach can produce viable candidates in weeks rather than years.
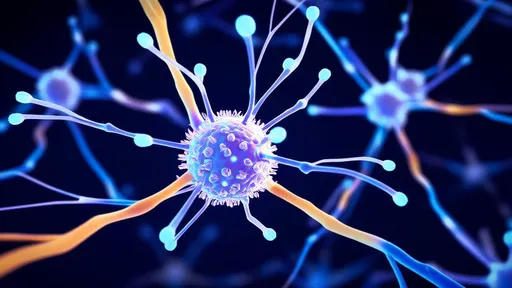
The most promising designs incorporate multiple functional domains - one for BBB recognition and transport, another for target cell binding, and a third for controlled cargo release. This modular architecture allows customization for different therapeutic applications while maintaining structural stability in the bloodstream. Early animal studies show some AI-designed carriers achieving brain concentrations 10-15 times higher than conventional delivery methods, with significantly reduced off-target accumulation.
What makes these protein carriers particularly remarkable is their dynamic responsiveness to the brain's microenvironment. Some variants remain inert during circulation, only activating their transport mechanisms upon encountering specific biochemical signals at the BBB interface. Others can temporarily disrupt tight junctions between endothelial cells just enough to permit passage, then facilitate their natural resealing. Such precision minimizes the risk of chronic barrier compromise that plagues many existing delivery techniques.
The development process represents a paradigm shift in biopharmaceutical design. Where traditional protein engineering relies on incremental modifications to known scaffolds, the AI system explores vast regions of theoretical protein space unconstrained by evolutionary precedent. This has yielded several completely novel folds with unexpected but highly effective BBB-interaction profiles. Researchers emphasize that these aren't random combinations, but carefully optimized structures meeting multiple criteria simultaneously - stability, specificity, and manufacturability.
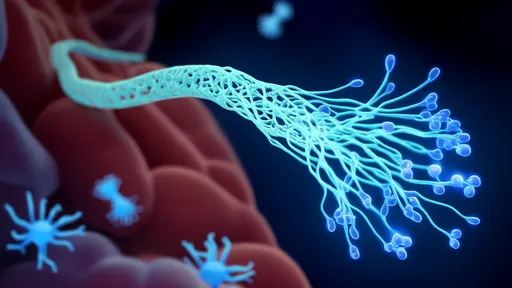
Clinical applications appear increasingly viable as the technology matures. Neurodegenerative diseases stand to benefit particularly, where the inability to deliver therapeutic antibodies or gene-editing tools has stalled numerous promising treatments. Early-stage collaborations with pharmaceutical companies are already adapting the carriers for anti-amyloid antibodies in Alzheimer's models and dopamine-regulating enzymes for Parkinson's. The approach also shows potential for delivering chemotherapy agents across the BBB in brain tumor cases, where precision targeting could dramatically reduce systemic toxicity.
Regulatory challenges remain significant, as these engineered proteins represent an entirely new class of biologic delivery vehicles. Researchers are compiling extensive safety data addressing potential immunogenicity and long-term effects on BBB integrity. However, the programmability of these systems allows for rapid iteration to meet safety requirements without compromising functionality. Some versions now in development incorporate human-derived protein sequences to minimize immune recognition.
Looking ahead, the technology's architects envision expanding beyond the BBB to other biological barriers that limit drug effectiveness. Similar AI platforms could design carriers targeting the placental barrier, the retinal barrier, or selective organ membranes. The underlying machine learning models grow more sophisticated with each new protein iteration, creating a virtuous cycle of improvement. As one lead researcher noted, "We're not just building better keys - we're learning the fundamental language of biological access."
The convergence of structural biology, artificial intelligence, and precision medicine embodied in this work suggests a near future where drug delivery challenges become design problems rather than impassable obstacles. With several AI-designed carriers expected to enter clinical trials within two years, the medical community watches closely. Success could establish a new template for overcoming nature's most stubborn biological defenses through computational creativity.
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025