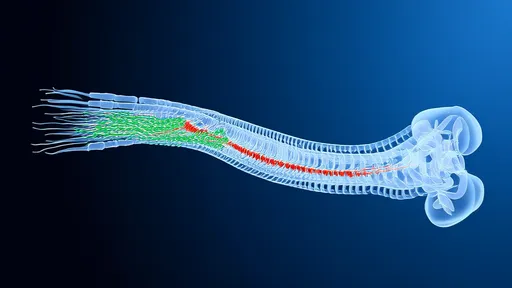
In a groundbreaking development that could revolutionize nerve repair, scientists have pioneered a novel approach using hydrogel optical fibers to guide the regeneration of damaged nerves. This innovative technique, often referred to as "neural photoconductive repair," merges the principles of optogenetics with advanced biomaterials to create a scaffold that not only supports nerve growth but also directs it with unprecedented precision. The implications for patients suffering from traumatic nerve injuries or neurodegenerative diseases are profound, offering hope where traditional treatments have fallen short.
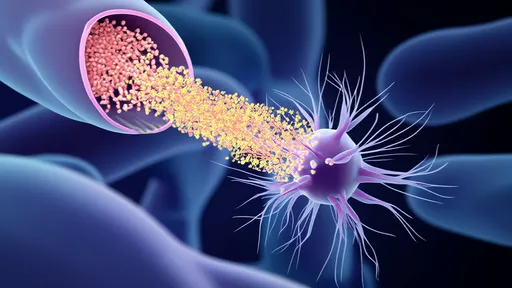
The core of this technology lies in the unique properties of hydrogel-based optical fibers. Unlike conventional nerve guides, these fibers are soft, flexible, and biocompatible, mimicking the natural environment of neural tissue. When implanted at the site of injury, they serve as both a physical conduit and a light-delivery system. Researchers have engineered these hydrogels to respond to specific wavelengths of light, enabling targeted stimulation of genetically modified neurons. This dual functionality—structural support and optogenetic control—creates a dynamic system where nerve regeneration can be actively guided rather than left to chance.
Early experimental results have been nothing short of remarkable. In animal models with severed sciatic nerves, the hydrogel光纤 approach demonstrated a 40% faster regeneration rate compared to standard nerve grafts. What's particularly striking is how the technology addresses one of the greatest challenges in nerve repair: the precise reconnection of proximal and distal nerve endings. The light-guided system appears to reduce misdirected axonal growth, a common issue that often leads to incomplete recovery or painful neuromas. Surgeons note that the transparency of the hydrogel allows for real-time visualization during implantation, a significant advantage over opaque synthetic conduits.
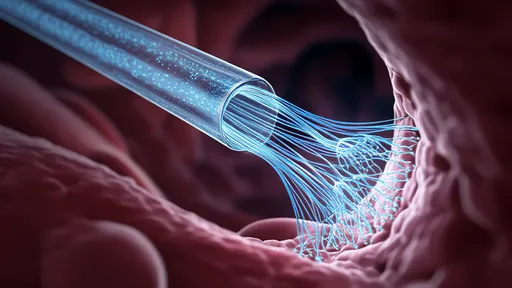
The development builds upon decades of research in both optogenetics and regenerative medicine. Scientists had previously established that certain wavelengths of light could stimulate neural activity in genetically modified cells. However, delivering light deep into tissues without causing damage remained a hurdle. Hydrogel光纤 solve this problem by acting as waveguides that can transmit light over centimeter-scale distances with minimal scattering or heat generation. Their water-rich composition (typically 85-90% water by weight) makes them exceptionally compatible with biological systems, reducing inflammatory responses that often hinder traditional implants.
Perhaps most intriguing is the technology's potential for adaptive therapy. Unlike static nerve guides, the optical properties of these hydrogels can be tuned post-implantation. Clinicians could theoretically adjust light parameters—intensity, pulse frequency, or wavelength—to optimize regeneration as the healing process progresses. This level of control opens possibilities for personalized treatment protocols based on individual patient responses. Some research teams are even exploring incorporating sensors into the hydrogels to create closed-loop systems that automatically adjust stimulation based on real-time neural activity.
Manufacturing these hydrogel光纤 requires sophisticated techniques borrowed from both microfabrication and biomaterial science. Researchers use a process called "photolithographic patterning" to create microscopic channels within the hydrogel matrix that function as optical waveguides. The materials are typically derived from natural polymers like hyaluronic acid or gelatin methacrylate, modified to achieve the right balance of mechanical strength and optical clarity. Recent advances have enabled the creation of fibers with multiple parallel channels, allowing for complex stimulation patterns that could potentially guide the regeneration of entire nerve bundles rather than single axons.
While the technology shows immense promise, significant challenges remain before clinical translation can occur. Long-term stability of the optical properties, potential immune responses to chronic light stimulation, and the ethical considerations of genetic modifications in human neurons all require careful study. Regulatory pathways for such combination products—part medical device, part biologic, part gene therapy—are still being defined. Nevertheless, with several biotech companies already investing in the approach and preliminary human trials anticipated within 3-5 years, neural photoconductive repair may soon transition from laboratory curiosity to clinical reality.
The broader implications extend beyond peripheral nerve repair. Researchers speculate that with further development, similar approaches could be applied to spinal cord injuries or even neurodegenerative conditions like Parkinson's disease. The ability to precisely guide neural growth while simultaneously modulating activity patterns could open new frontiers in neuroregeneration. As one lead researcher remarked, "We're not just repairing nerves—we're creating smart scaffolds that actively participate in the healing process." This paradigm shift from passive to active regeneration technologies may well define the next era of neurological medicine.
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025