In a groundbreaking study that reshapes our understanding of brain metabolism, researchers have uncovered the pivotal role of glial cells as "energy stewards" in the nervous system. The findings reveal an intricate molecular dialogue between astrocytes and neurons that governs the distribution of mitochondria—the cellular powerhouses—to meet localized energy demands. This discovery challenges the long-held neuron-centric view of brain energetics, painting glia as active architects of neural metabolic networks rather than passive support cells.
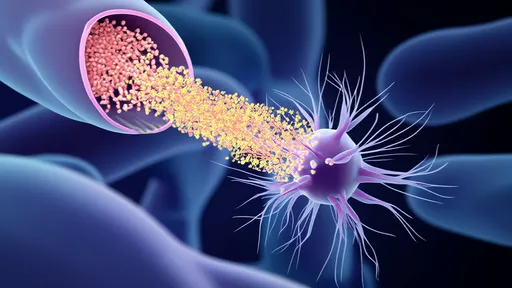
The research demonstrates how astrocytes employ calcium signaling to detect neuronal activity patterns and subsequently regulate mitochondrial transfer to high-activity zones. What makes this mechanism remarkable is its precision—glia don't simply flood neurons with mitochondria, but strategically allocate these organelles based on real-time computational needs. This process involves the coordinated action of adhesion molecules, tunneling nanotubes, and vesicular transport systems that together form a responsive energy distribution network.
Scientists observed that during periods of sustained cognitive processing, astrocytes preferentially shuttle mitochondria to dendritic spines engaged in active synaptic plasticity. This targeted delivery correlates with enhanced synaptic strength and improved signal transmission efficiency. The study provides concrete evidence that memory formation isn't just about strengthening connections between neurons, but equally depends on the astrocyte-mediated optimization of energy resources at these connection points.
Pathological implications are profound. In mouse models of neurodegenerative diseases, researchers found that disrupting glial control of mitochondrial distribution accelerated neuronal dysfunction. The energy crisis in affected neurons appeared earlier and more severely when astrocytes couldn't properly allocate mitochondria, suggesting that glial metabolic management fails before classical pathological markers emerge. This insight could redirect therapeutic strategies toward supporting glial function in early disease stages.
Advanced imaging techniques revealed the dynamic nature of this regulatory system. Time-lapse microscopy showed mitochondria pausing at specific locations along neuronal processes, apparently awaiting "permission" from nearby astrocytes to proceed to their destinations. Such observations imply the existence of molecular checkpoints where glial cells evaluate and authorize mitochondrial passage—a level of control previously unrecognized in neural metabolism.
The study also uncovered surprising heterogeneity among astrocyte populations. Not all glial cells participate equally in mitochondrial regulation; distinct subtypes appear specialized for servicing particular neuronal circuits or brain regions. This specialization mirrors the functional diversity of neurons themselves, suggesting that the brain maintains dedicated energy management teams for different computational tasks.
From an evolutionary perspective, the findings help explain how complex brains achieve metabolic efficiency. As neural networks grew more sophisticated, simply increasing the number of mitochondria in every neuron would have been energetically unsustainable. The glial-mediated allocation system allows for strategic energy investment where and when it's needed most, enabling higher cognitive functions without prohibitive metabolic costs.
Practical applications are already emerging. Researchers successfully enhanced learning in rodents by experimentally boosting glial control of mitochondrial distribution. This was achieved not by increasing overall mitochondrial production, but by improving the precision of their deployment. Such approaches might eventually lead to cognitive enhancement therapies that work by optimizing rather than maximising brain energy use.
The discovery also solves longstanding puzzles about regional vulnerabilities in neurological disorders. Brain areas with particularly high or fluctuating energy demands appear most dependent on glial metabolic regulation, making them first to falter when this system breaks down. This explains why diseases like Alzheimer's show characteristic patterns of degeneration rather than uniform neuronal loss.
Looking ahead, the research team is exploring how other glial cell types participate in the brain's energy economy. Early data suggest oligodendrocytes and microglia may play complementary roles in mitochondrial quality control and recycling, pointing to an integrated glial network that sustains neuronal metabolism on multiple timescales. These insights promise to revolutionize our conception of brain health and disease.
As the scientific community digests these findings, textbooks are being rewritten to reflect glia's active role in information processing. No longer mere "glue" holding neurons together, these cells emerge as sophisticated managers of the brain's energy budget—a revelation with far-reaching consequences for neuroscience and medicine. The study establishes a new paradigm where cognition arises not just from neuronal activity, but from the intricate metabolic partnership between neurons and their glial stewards.
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025