In a groundbreaking development that could reshape cancer treatment paradigms, researchers have unveiled a novel biomimetic material designed to mimic the tumor microenvironment and keep metastatic cells in a dormant state. Dubbed the "dormancy lock," this approach represents a radical departure from traditional therapies that aggressively target proliferating cancer cells, instead focusing on controlling the deadly process of metastasis responsible for 90% of cancer-related deaths.
The science behind this innovation stems from decades of research into why some cancer cells remain dormant for years before suddenly awakening to form lethal secondary tumors. By recreating key aspects of the native tumor niche that naturally suppress cell proliferation, scientists have engineered smart materials that essentially trick disseminated tumor cells into maintaining quiescence indefinitely.
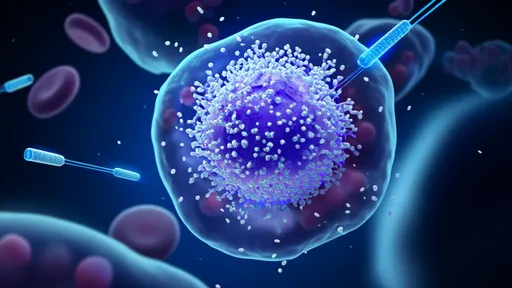
What makes this biomaterial platform particularly remarkable is its dual-action mechanism. The scaffold not only provides physical and biochemical cues to maintain dormancy but also contains sensors that detect when cells begin showing signs of activation. When such awakening is detected, the material releases targeted inhibitors to push cells back into quiescence—creating what researchers describe as a "virtuous cycle" of dormancy reinforcement.
Early animal studies have shown unprecedented results. In models of breast cancer metastasis, the implantable material reduced metastatic outgrowth by 87% compared to control groups. Perhaps more impressively, the treated animals showed no signs of the liver and lung metastases that typically develop in these models, even six months post-treatment.
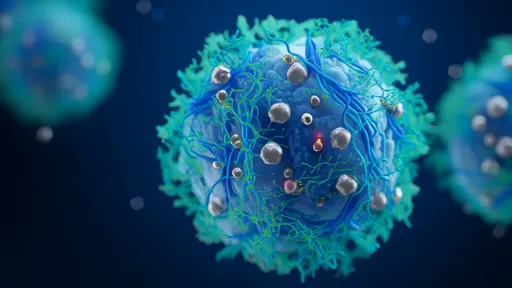
The key breakthrough came from decoding the precise extracellular matrix composition and mechanical properties that keep disseminated tumor cells in check. Through painstaking analysis of the bone marrow microenvironment—where many cancer cells hide in dormancy—the team identified a specific combination of collagen density, fibronectin patterning, and stiffness thresholds that collectively suppress proliferation signals.
Clinical translation efforts are already underway, with first-in-human trials expected to begin within two years. The material can be delivered as an injectable hydrogel that solidifies at the target site, making it suitable for both preventive applications in high-risk patients and therapeutic use in early-stage metastasis scenarios. Unlike systemic therapies that cause widespread side effects, this localized approach minimizes toxicity while maximizing impact on dangerous micrometastases.
Oncologists not involved in the research have expressed cautious optimism. Dr. Elena Rodriguez from Memorial Sloan Kettering noted, "If these findings hold up in clinical trials, we could be looking at a fundamental shift in how we manage cancer. Instead of waiting for metastases to appear then scrambling to treat them, we might soon have tools to keep them permanently dormant—effectively making cancer a chronic but controllable condition."
The research team is now exploring combination strategies, particularly how the dormancy lock might work synergistically with immunotherapy. Preliminary data suggest that maintaining tumor cells in quiescence could make them more vulnerable to immune recognition, potentially allowing the body's natural defenses to eliminate dormant cells that would otherwise be invisible to surveillance mechanisms.
Beyond its immediate clinical potential, this work provides profound new insights into cancer biology. By reverse-engineering the natural processes that maintain cellular dormancy, scientists have uncovered previously unknown pathways and checkpoints that could lead to additional therapeutic targets. The biomaterial platform itself serves as a powerful research tool for studying the complex interplay between disseminated tumor cells and their microenvironment.
As with any emerging technology, challenges remain. Questions about long-term material stability, the potential for late escape mechanisms, and optimal delivery protocols need addressing. Yet the scientific community largely agrees this represents one of the most promising anti-metastasis strategies to emerge in recent years—one that might finally turn the tide against cancer's deadliest phase.
Looking ahead, researchers envision a future where dormancy-inducing materials become standard components of cancer care, used alongside surgery, radiation, and traditional drugs to provide comprehensive protection against recurrence and spread. For millions of cancer patients living with the sword of Damocles that is metastatic risk, such advances can't come soon enough.
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025