In a groundbreaking development that could revolutionize cellular medicine, scientists have pioneered a novel approach to mitochondrial therapy using engineered exosomes as delivery vehicles for healthy mitochondria. This innovative strategy, often referred to as "mitochondrial charging stations," addresses the root cause of numerous degenerative diseases by replenishing damaged cellular power plants with functional counterparts.
The concept builds upon our growing understanding of mitochondria as more than just energy producers—these organelles serve as metabolic hubs, calcium regulators, and apoptosis mediators. When mitochondria malfunction, cells experience catastrophic energy failures that manifest as neurological disorders, cardiovascular diseases, and accelerated aging. Traditional pharmacological interventions often fail because they don't replace the damaged organelles themselves.
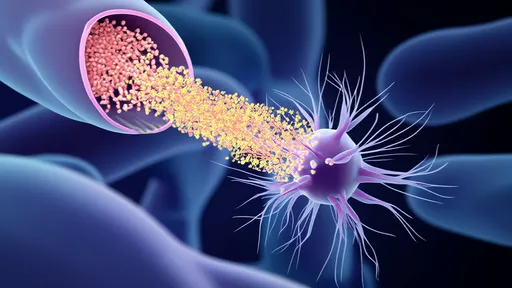
Exosomes—nature's perfect delivery system—have emerged as the unexpected heroes in this mitochondrial transfer narrative. These naturally occurring extracellular vesicles, typically 30-150 nanometers in diameter, evolved as intercellular communication vehicles. Researchers have now harnessed their biocompatibility, stability, and targeting capabilities to create mitochondrial ferries capable of navigating the bloodstream and precisely delivering their cargo to compromised cells.
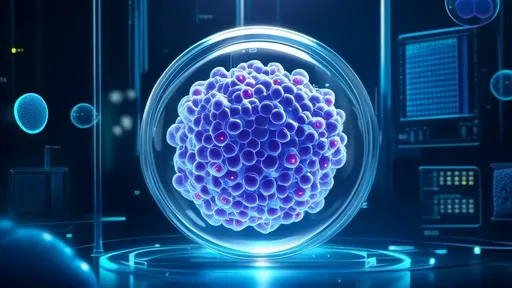
The engineering process begins with donor cells that package healthy mitochondria into exosomes through a specialized budding process. Scientists enhance this natural loading mechanism through electrical stimulation and membrane modification techniques. The resulting mitochondrial-loaded exosomes (MExos) exhibit remarkable preservation of mitochondrial membrane potential and oxidative phosphorylation capacity—essential for their therapeutic efficacy.
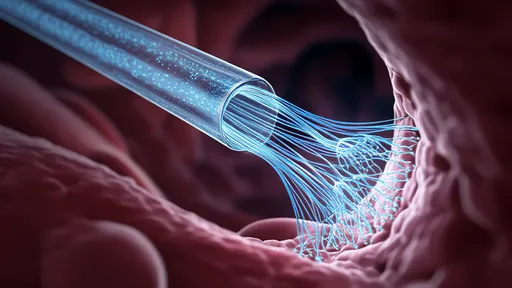
What makes this approach particularly promising is its ability to overcome the historic challenges of mitochondrial transplantation. Previous attempts using direct injection or viral vectors faced immune rejection, poor targeting, and mitochondrial degradation during transit. The exosomal packaging protects mitochondria from immune surveillance while providing natural homing capabilities to specific tissues based on surface markers.
Early preclinical results have demonstrated astonishing recovery in animal models of Parkinson's disease. Dopaminergic neurons, which are particularly vulnerable to mitochondrial dysfunction, showed significant metabolic recovery after MExo treatment. The exosomes successfully crossed the blood-brain barrier—a feat that most drug delivery systems struggle to achieve—and delivered functional mitochondria to neurons in affected brain regions.
The cardiovascular applications appear equally promising. In myocardial infarction models, engineered exosomes preferentially homed to ischemic heart tissue and integrated their mitochondrial cargo into cardiomyocytes. Treated animals exhibited improved ejection fraction and reduced scar tissue formation compared to controls. Researchers speculate this approach might eventually prevent heart failure progression after heart attacks.
Beyond disease treatment, the technology shows potential for combating age-related mitochondrial decline. As we age, our mitochondria accumulate mutations and become less efficient—a primary contributor to frailty and organ dysfunction. Periodic MExo "boosters" could theoretically maintain cellular energy production at youthful levels, potentially extending healthspan.
The manufacturing process presents interesting scalability advantages. Donor cells can be cultured to produce large quantities of mitochondrial exosomes, and the vesicles can be frozen for long-term storage without losing potency. This differs from whole-cell therapies that often require fresh preparation and complex logistics.
Safety considerations remain paramount as the technology progresses toward human trials. Researchers are carefully evaluating potential risks including off-target effects, immune reactions to donor mitochondria, and the theoretical possibility of horizontal gene transfer. Early data suggests these risks may be lower with exosomal delivery compared to other mitochondrial transfer methods, but comprehensive long-term studies are underway.
Ethical discussions have emerged regarding the source of donor mitochondria. While most current research uses mitochondria from mesenchymal stem cells, some propose using patient-specific induced pluripotent stem cells to create perfectly matched mitochondrial donors. Others advocate for universal donor cell lines that could be banked for widespread use.
The business landscape is responding enthusiastically to these scientific advances. Several biotech startups have already secured substantial funding to develop mitochondrial exosome platforms. Pharmaceutical giants are establishing partnerships to explore combination therapies—pairing traditional drugs with mitochondrial restoration for synergistic effects in complex diseases.
Regulatory agencies face novel challenges in evaluating this hybrid therapy—part drug, part organelle transplant. The FDA has convened special committees to develop appropriate frameworks for assessing mitochondrial therapies, recognizing they don't fit neatly into existing categories of biologics or medical devices.
Looking ahead, researchers envision a future where mitochondrial "charging stations" become as commonplace as dialysis centers. Patients might receive periodic mitochondrial replenishment for chronic conditions, or emergency mitochondrial transfusions after events like strokes or heart attacks. The approach could potentially be combined with gene editing to correct mitochondrial DNA mutations during the transfer process.
As the field progresses, scientists are exploring ways to enhance exosome targeting precision through surface modifications. Some teams are engineering exosomes that respond to specific metabolic signals, only releasing their mitochondrial cargo when they detect the biochemical signature of distressed cells. Others are developing "smart" exosomes that can deliver different mitochondrial subtypes tailored to specific cellular needs.
The implications for rare mitochondrial diseases are particularly profound. These often-fatal genetic disorders, caused by mutations in mitochondrial DNA, currently have no cure. MExo therapy offers hope for replacing the entire population of defective mitochondria in affected tissues—a feat impossible with current pharmacological approaches.
While significant challenges remain before clinical application, the convergence of exosome biology and mitochondrial medicine marks a paradigm shift in how we approach cellular energy disorders. This elegant solution, inspired by nature's own delivery systems, may soon transform our ability to repair the fundamental power sources of life itself.
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 7, 2025